By Windsor Aguirre
Fishes are extremely diverse and exceed all other living vertebrate groups combined in terms of species numbers. With this great taxonomic diversity comes a great diversity of forms. From seahorses and hatchet fishes to eels and the common mola, from tiny gobies and miniature tetras to giant groupers and the great pirarucu, fishes come in all shapes and sizes (Fig. 1).
Fig. 1. A. Common mola (courtesy of Factzoo.com). B. Adult male miniature tetra Iotabrycon praecox from western Ecuador. White scale bar is 10mm (from Aguirre et al., 2014a). C. Pirarucu (courtesy of Rivermonsters.com).
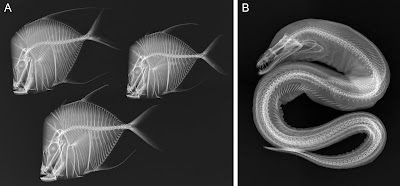
A major theme in the variability of fish form is the evolution of elongation. Extreme elongation has evolved repeatedly in different groups of fishes (and other vertebrates, for that matter), and is strongly associated with changes in the axial skeleton (Fig. 2), especially increases in the number of vertebrae (e.g., Ward & Brainerd, 2007; Ward & Mehta, 2010). Advances in evo-devo are facilitating the study of the molecular mechanisms responsible for changes in body form and axial patterning. For example, there has been great progress on deciphering the molecular mechanisms through which the number of body segments is regulated and the identity of body segments is established in vertebrates (Gomez et al., 2008; Gomez & Pourquié, 2009; Mallo et al., 2010). Moreover, several recent studies present candidate genes for the evolution of vertebral number in fishes (e.g., Ward & Mehta, 2010; Kimura et al., 2012; Berner et al., 2014). Although much remains to be learned, progress is definitely being made.
Fig. 2. Radiographs showing examples of variation in body form and vertebral number in fishes (courtesy of the Smithsonian Institution).
But what about the early stages of the evolution of body elongation? What are the selective pressures at play early on that guide fish with typical body forms to evolve body elongation? How do the structural changes originate, how are developmental pathways modified, and what are the functional consequences along the way? That old friend of evolutionary biologists, the threespine stickleback (Gasterosteus aculeatus), may provide some insights.
I have been studying the threespine stickleback since the early 2000’s, when my former Ph.D. advisor, Mike Bell, formally introduced me to them. Although there are many aspects of their morphology that have attracted the interest of scientists, body shape has been a standout. Body shape variation is substantial within this species and correlates strongly with ecology. Oceanic ancestral populations differ greatly from the resident freshwater populations that they have established throughout the world. Resident freshwater populations also differ substantially from one another. Along one axis of diversification in freshwater environments, resident populations in streams and shallow lakes (benthics) evolve relatively deep bodies, whereas resident populations in deep lakes (limnetics) evolve more elongated, streamlined bodies. This shape variation has been characterized repeatedly in different geographic areas and is relatively well understood (e.g., Walker, 1997; Spoljaric & Reimchen, 2007; Aguirre, 2009). Is this variation in body form at the microevolutionary level associated with changes in the axial skeleton as seen across broad taxonomic ranks in fishes? Does vertebral number increase in more elongate limnetics?
Since this is the threespine stickleback we are talking about, an evolutionary “supermodel” (Gibson, 2005), there is of course some previous research on the subject that suggests that body form indeed covaries with variation in the axial skeleton in the predicted direction (e.g., Reimchen and Nelson, 1987; Ahn, 1988). To explore the evolution of body form and vertebral architecture in stickleback in greater detail, my students and I set up a study to address four basic questions:
- Is there sexual dimorphism in vertebral number? Stickleback are sexually dimorphic for many traits, and males and females can experience very different selective demands. They are also known to differ significantly in body form, and Reimchen and Nelson (1987) previously reported differences between sexes in total vertebral number, with males having more vertebrae.
- Is there a significant difference in vertebral number among anadromous (oceanic), benthic/stream, and limnetic populations of threespine stickleback? If so, we expected limnetics to have more vertebrae than the other ecomorphs.
- Is there body region specificity in terms of where vertebral number changes? Fish have two major types of vertebrae, abdominal (or precaudal) vertebrae and caudal vertebrae. These have different forms and serve different purposes, so it makes sense that where you add or subtract vertebrae may matter functionally.
- Finally, is there an association between vertebral number and body shape within ecomorphs? That is,do more elongate individuals within a particular ecomorph tend to have more vertebrae than more deep-bodied individuals within the same ecomorph?
Body shape data were collected using geometric morphometric methods, which allow for very precise measurement and visualization of shape differences among specimens. We then took the same specimens to the Field Museum of Natural History (Chicago), where they were X-rayed to obtain vertebral number data (Fig. 3). Details of the methods of analysis are available in Aguirre et al. (2014b).
Fig. 3. Radiograph showing method for counting vertebral number.
Was there sexual dimorphism in vertebral number? We only have data on nine of the populations for this, but the result was consistent enough to draw a conclusion without the need to survey all 20 populations. Surprisingly, we did not detect a significant difference in total vertebral number between sexes as Reimchen and Nelson (1987) did. Geographic variation or differences in statistical power between studies may account for the difference. However, we did see a consistent and statistically significant difference in the proportion of abdominal to caudal vertebrae between the sexes, with females having more abdominal vertebrae and males having more caudal vertebrae (Fig. 4). The difference was not very large but the trend was the same in every population for which data were collected. This is consistent with a transformation of vertebral identities resulting in an expansion in the abdominal region in females, perhaps to increase the volume available for packing eggs. Stickleback are small fish and the abdomen of gravid females can exhibit fairly extreme distension, suggesting that an expansion of the abdominal region in females might increase fitness by allowing them to pack a greater number of eggs in the abdomen. This was previously suggested by Lindsey (1962, 1975) and similar patterns have been reported for other types of fish (Hart & McHugh, 1944; Hastings, 1991), although it is not clear whether this is widespread in fishes.
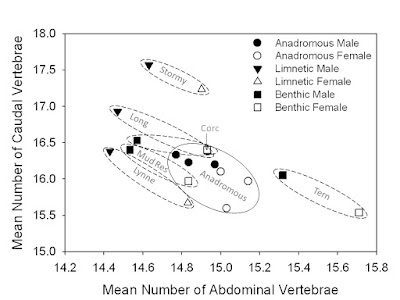
Fig. 4. Sexual dimorphism in vertebral number among Alaskan threespine stickleback. Mean number of abdominal vertebrae is plotted against mean number of caudal vertebrae, coded by sex for the nine populations for which data are available. Note the consistent pattern of divergence between sexes. In every population males have more caudal vertebrae and fewer abdominal vertebrae than females, although total vertebral number did not differ significantly between sexes.
Is there a significant difference in vertebral number among anadromous (oceanic), benthic/stream, and limnetic populations of threespine stickleback? Yes. Using samples of males from all 20 populations surveyed, limnetics have significantly more vertebrae than populations of the other ecomorphs (Fig. 5A). The difference is only 0.5-0.6 more vertebrae on average – not very large, but statistically significant and fairly consistent with the exception of one limnetic population, Lynne Lake, which was an outlier for every variable measured. Whether vertebrae are changing in length as well is something that we are currently examining. There was also fairly obvious body region specificity in terms of where vertebral number changed. It was the number of caudal vertebrae that increased in limnetics and accounted for the greater number of vertebrae overall. This is consistent with the previously documented expansion of the caudal region of the body in limnetics, suggesting covariation between body form and vertebral number as observed at broader taxonomic ranks.
Fig. 5. Vertebral number variation among male Alaskan stickleback. Points are population sample means. A) Variation in mean total vertebral number. B) The mean number of abdominal vertebrae plotted against the mean number of caudal vertebrae. Notice that limnetics have more vertebrae than the other ecomorphs and that it is caudal vertebral number that specifically increases in limnetics.
Finally, is there an association between vertebral number and body shape within ecomorphs? Again, the answer is yes. We conducted a discriminant function analysis (DFA) on the body shape data to classify individuals as being relatively benthic-/stream-shaped (treating benthic lake and stream populations as one ecomorph because of their morphological and ecological similarity) or relatively limnetic-shaped, and then calculated the mean DF score along this shape-type axis for each population. Populations with more extreme mean scores along the axis had more pronounced benthic/stream (positive side) or limnetic (negative side) body shapes. Mean population DF score based on the body shape data was strongly correlated with total vertebral number within both ecomorphs (r = -0.888 and -0.811 for benthic/stream populations and limnetic populations, respectively), indicating that body shape was correlated with variation in vertebral number in the same way within both ecomorphs (Fig. 6).
Fig. 6. Association between total vertebral number and body shape within ecomorphs. Anadromous samples not included, and benthic and stream samples pooled as one ecomorph.
Where do we go from here?
A major goal of future research will be to try to decipher whether variation in vertebral number matters functionally at this scale. Vertebral number and body shape covary in stickleback, so it is possible that differences in vertebral number per se do not matter functionally; perhaps the trait being selected for is the change in body shape, with selection for more elongate bodies and an expanded caudal region in limnetics resulting in a correlated increase in the number of caudal vertebrae. However, maybe the number of vertebrae itself does matter. Inasmuch as increasing the number of vertebrae, for fish with similar body shapes, may result in an increase in flexibility, changes in vertebral number may impact burst-swimming performance since rapidly bending the body is an important part of the escape response of fishes. This was suggested previously by Brainerd and Patek (1998), although much more work on this issue is needed, including in the threespine stickleback.
How are differences in vertebral number associated with vertebral length? Vertebral length decreases from the anterior to the posterior region of the vertebral axis (unpublished data), suggesting that more and shorter vertebrae in the caudal region is functionally advantageous. Are there patterns to how vertebral length changes in fish differing in vertebral number? Are there uniform changes throughout the body axis, or does there tend to be body region specificity in how vertebral lengths are modified?
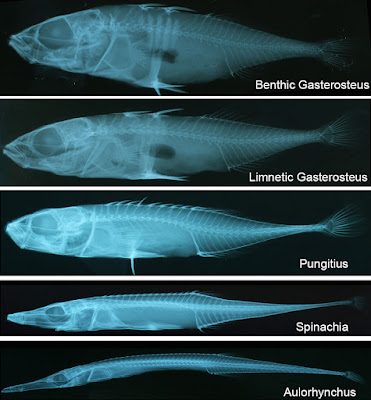
Finally, do these small changes in vertebral number really matter beyond the threespine stickleback? How do we connect the results from this study to the evolution of extreme body elongation in vertebrates? This is a trickier question, but a potential direction for future research is suggested when one looks at variation among the threespine’s relatives. Gasterosteus aculeatus is not the only stickleback. There are other genera of stickleback, and – as is the case across fishes – body elongation is a major theme in the evolution of body form among the Gasterosteidae and their closest relatives (Fig. 7). The ninespine stickleback, Pungitius, is more elongate than Gasterosteus, and this elongation is associated with a slight increase in vertebral number. Then there is the fifteenspine stickleback, Spinachia spinachia, which exhibits extreme body elongation. A beautiful fish with a remarkable body form, it looks like the cartoon stick-figure version of a stickleback, and exhibits a substantial increase in vertebral number. Continue to the closest known relatives of the gasterosteids, Aulorhynchus and Aulichthys, and even more extreme elongation and greater increases in vertebral number are observed. How are these extreme changes in morphology achieved? How did extreme elongation evolve in these fishes? Can comparative analyses across these related lineages differing in body elongation and vertebral phenotypes illuminate our understanding of the evolution of vertebrate body form? Time will tell…
Fig. 7. Radiograph illustrating phenotypic variation associated with elongation in gasterosteids (Gasterosteus, Pungitius, and Spinachia) and their relatives (Aulorhynchus).
Literature Cited:
- Aguirre, W.E. 2009. Microgeographical diversification of threespine stickleback: body shape-habitat correlations in a small, ecologically diverse Alaskan drainage. Biological Journal of the Linnean Society 98:139-151.
- Aguirre, W.E., R. Navarrete, P. Calle, and G.C. Sanchez-Garces. 2014a. First Record of Iotabrycon praecox Roberts 1973 (Characidae) in the Santa Rosa River, southwestern Ecuador. Checklist 10:382-385.
- Aguirre, W.E., Walker, K., Gideon, S. 2014b. Tinkering with the axial skeleton: vertebral number variation in ecologically divergent threespine stickleback populations. Biological Journal of the Linnean Society 113:204-219.
- Ahn, D. 1998. Factors controlling axial variation in the threespine stickleback, Gasterosteus aculeatus (Teleostei: Gasterosteidae): pattern of natural variation and genetic/developmental mechanisms. DPhil Thesis, University of Michigan.
- Berner, D., Moser, D., Roesti, M., Buescher, H., Salzburger, W. 2014. Genetic architecture of skeletal evolution in European lake and stream stickleback. Evolution 68: 1792–1805.
- Brainerd, E.L., Patek, S.N. 1998. Vertebral column morphology, C-start curvature, and the evolution of mechanical defenses in tetraodontiform fishes. Copeia 1998: 971–984.
- Gibson, G. 2005. The synthesis and evolution of a supermodel. Science 307:1890-1891.
- Gomez, C., Ozbudak, E.M., Wunderlich, J., Baumann, D., Lewis, J., Pourquié, O. 2008. Control of segment number in vertebrate embryos. Nature 454: 335–339.
- Gomez, C., Pourquié, O. 2009. Developmental control of segment numbers in vertebrates. Journal of Experimental Zoology 312B: 533–544.
- Hart, J.L., McHugh, J.L. 1944. The smelts (Osmeridae) of British Columbia. Bulletin of the Fisheries Research Board of Canada 64: 1–27.
- Hastings, P.A. 1991. Ontogeny of sexual dimorphism in angel blenny, Coralliozetus angelica (Blennioidei:Chaenopsidae). Copeia 1991: 969–978.
- Kimura, T., Shinya, M., Naruse, K. 2012. Genetic analysis of vertebral regionalization and number in medaka (Oryzias latipes) inbred lines. G3: Genes, Genomes, Genetics 2: 1317–1323.
- Lindsey, C.C. 1962. Experimental study of meristic variation in a population of threespine sticklebacks, Gasterosteus aculeatus. Canadian Journal of Zoology 40: 271–312.
- Lindsey, C.C. 1975. Pleomerism, the widespread tendency among related fish species for vertebral number to be correlated with maximum body length. Journal of the Fisheries Research Board of Canada 32: 2453–2469.
- Mallo, M., Wellik, D.M., Deschamps, J. 2010. Hox genes and regional patterning of the vertebrate body plan. Developmental Biology 344: 7–15.
- Spoljaric, M.A., Reimchen, T.E. 2007. 10 000 years later: evolution of body shape in Haida Gwaii three-spined stickleback. Journal of Fish Biology 70: 1484–1503.
- Walker, J.A. 1997. Ecological morphology of lacustrine threespine stickleback Gasterosteus aculeatus L. (Gasterosteidae) body shape. Biological Journal of the Linnean Society 61: 3–50.
- Ward, A.B., Brainerd, E.L. 2007. Evolution of axial patterning in elongate fish. Biological Journal of the Linnean Society 90: 97–116.
- Ward, A.B., Mehta, R.S. 2010. Axial elongation in fishes: using morphological approaches to elucidate developmental mechanisms in studying body shape. Integrative and Comparative Biology 50: 1106–1119.